We Are Newborn Screening

September is Newborn Screening Awareness Month
 dried blood spot, testing for 55 genetic disorders
dried blood spot, testing for 55 genetic disorders hearing screening
hearing screening critical congenital heart disease screening
critical congenital heart disease screening
This year, we are shining a spotlight on the incredible teams working behind the scenes to ensure every baby born in Texas gets the care they need.
We encourage you to recognize your team and all the work they put into making NBS successful in Texas.
Pre-Testing Teams
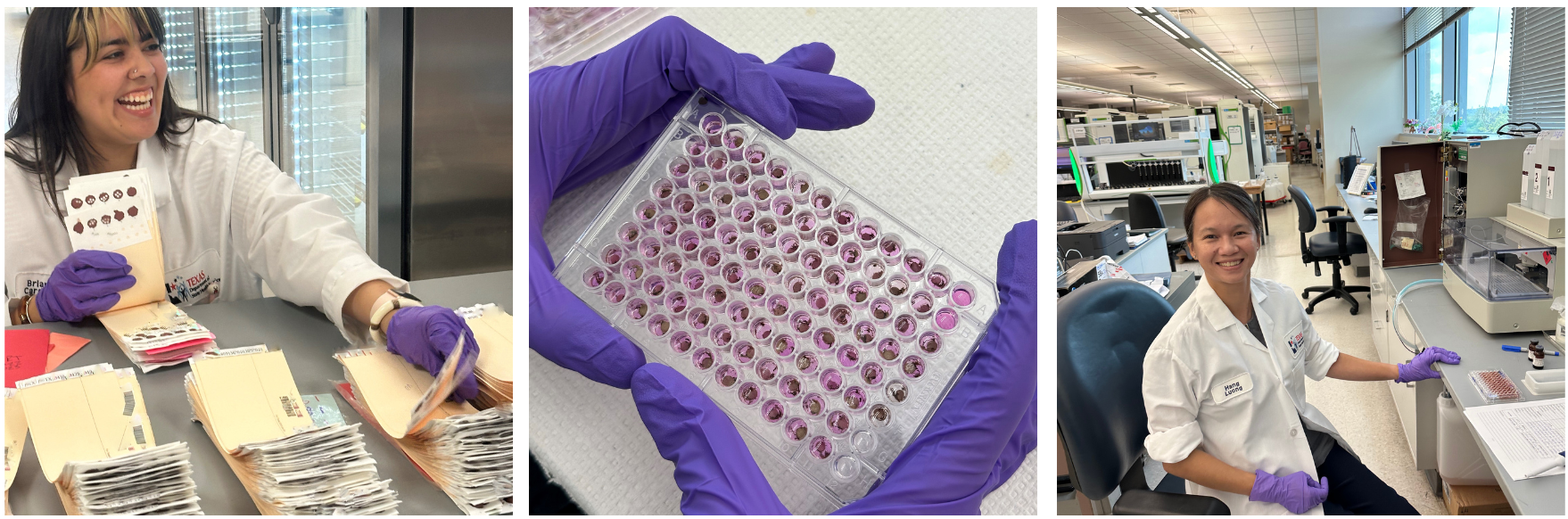
Check-in: Processing One Specimen at a Time
The Newborn Screening (NBS) Check-in Team is vital to the newborn screening process. They process each specimen efficiently and accurately. This group of 12 team members handles 2,000 to 3,000 specimens per day, six days a week. 
The Check-in Team opens all mail and parcels that contain newborn screening specimens delivered to the Laboratory. Once opened, each newborn screening kit gets scanned into our laboratory information management system (LIMS) to register the date of receipt at the Laboratory. Check-in sorts the specimens by the age of the baby at time of collection. Specimens less than 7 days of age get processed as first screen specimens. Specimens greater than 7 days of age get processed as second screen specimens.
 The Check-in Team then does a quality review. They look for missing or inconsistent demographic information. They also assess the quality of the specimen. It is important to remember that specimens must arrive in the Laboratory within thirteen days of collection to be viable for testing.
The Check-in Team then does a quality review. They look for missing or inconsistent demographic information. They also assess the quality of the specimen. It is important to remember that specimens must arrive in the Laboratory within thirteen days of collection to be viable for testing.
After this review, each specimen gets stamped with a unique identification number. This unique identification number links to the kit’s serial number in the LIMS. Next, the specimen gets separated from the demographic information form. Check-in scans the demographic form and attaches it to the kit’s serial number for reference in the LIMS system. The demographic information form is then given to the Reporting Team to complete data entry.
From NBS Check-in, specimens get directed to one of three areas:
-
Those missing key demographic information go to the Specimen Logistics Team for follow-up.
-
Specimens with quality issues get sent to testing staff for further review.
-
Those in good condition and have all key demographic information proceed to Specimen Preparation for the next stage of processing.
The Check-in Team’s goal is to have all specimens ready for testing by 2:30 p.m. each day. The Check-in Team is an essential component of the Laboratory’s success in providing timely and accurate newborn screening results.
Specimen Logistics: Ensuring the Flow of Specimens from Collection to Testing
The Newborn Screening (NBS) Laboratory Logistics Team plays a crucial role in managing the journey of specimens after they arrive at the Laboratory. This team handles transporting the specimens from Check-In to the Testing Lab. One of the team’s key functions is to promote a continuous yet smooth workflow.
 Ever wonder who sends the faxes requesting information? The Logistics Team has responsibility for contacting submitters to collect missing information. Resolving issues that would delay testing is important. This team also notifies submitters when specimens are not acceptable for testing. The team begins tracking recollections by linking the patient’s screening records together. Linking cases also assists with patient care and follow-up.
Ever wonder who sends the faxes requesting information? The Logistics Team has responsibility for contacting submitters to collect missing information. Resolving issues that would delay testing is important. This team also notifies submitters when specimens are not acceptable for testing. The team begins tracking recollections by linking the patient’s screening records together. Linking cases also assists with patient care and follow-up.
This team also processes the Parent Decision Form for Storage and Use of NBS Blood Spot Cards. Keeping or destroying the residual blood spots starts with receiving the form. Staff record the parent's decision in the Laboratory Information Management System (LIMS). The team also manages the destruction process according to the timelines established by the Texas Health and Safety Code.
The NBS Laboratory Logistics Team coordinates a complex operation involving many areas.
How Supply and Preparation Teams Power the Newborn Screening Program
Laboratory Supply Team
The Laboratory Supply Team, made up of three staff members, plays a vital role in supporting the Newborn Screening (NBS) Program. This team is responsible for fulfilling supply requests within the Laboratory, delivering the required items, and ensuring that the supplies—ranging from general consumables, like gloves, to crucial reagents—are always available. These items must always be available to meet testing needs.
 The team oversees the delivery of the NBS kits to the Laboratory, ensuring they arrive without damage and are stored in a safe, temperature-controlled environment, protected from any hazards that could compromise their quality.
The team oversees the delivery of the NBS kits to the Laboratory, ensuring they arrive without damage and are stored in a safe, temperature-controlled environment, protected from any hazards that could compromise their quality.
The Laboratory Supply Team’s work is vital to maintaining accurate testing schedules and supporting the Laboratory’s overall operations. Their efficiency ensures that the Laboratory runs smoothly, contributing to the successful processing and analysis of newborn screening specimens.
Container Preparation Team
The Container Preparation Team consists of five staff members who play a crucial role in the Newborn Screening (NBS) process. They fulfill requests for NBS kits that submitters use to collect specimens from babies and send to the Laboratory for testing. The team carefully reviews orders received via email or fax, checks order history to avoid duplicates, and contacts submitting facilities if any details need clarification.
Customer service is a key aspect of their role, as they are often the first point of contact for troubleshooting issues related to supply requests, billing holds, and shipment information related to submitter NBS kit requests. They also guide submitters to the correct program areas for assistance. Timely and accurate order processing is critical. Delays can hinder submitters' ability to perform essential NBS services for patients. The work of the Container Preparation Team is vital for maintaining the flow of specimen collection and testing, which begins with the proper supply of materials.

Precision in Every Punch: The Key to Accurate Newborn Screening
Upon receiving the dried blood spot specimens, Newborn Screening (NBS) Laboratory staff, for all testing areas, begin processing the specimens that arrive that day. The NBS testing process begins with punching a precise dried blood dot from the specimen. This step is crucial as it sets the stage for accurate and efficient analysis of each specimen.
Each specimen undergoes a carefully controlled procedure. An automated instrument cuts 3.2 mm dots from the dried blood spots, punching nine initial dots from each NBS specimen directly into 96-well microtiter plates. The instrument uses advanced software to punch the samples in a specific pattern, accommodating control material. Before punching, the staff conducts a final quality review to ensure that each initial screen is performed on a fully saturated dried blood spot.
 To maintain accurate identification throughout the process, the Laboratory uses a positive identification system. The punching instrument reads the barcode on each NBS specimen, captures an image of the specimen, and links the blood dot to its specific well location in the microtiter plates within the laboratory information management system.
To maintain accurate identification throughout the process, the Laboratory uses a positive identification system. The punching instrument reads the barcode on each NBS specimen, captures an image of the specimen, and links the blood dot to its specific well location in the microtiter plates within the laboratory information management system.
The punching process ensures accurate and efficient preparation of NBS specimens for testing. This process lets the lab keep high standards and provide reliable NBS results. These screening results help in the early detection and treatment of serious conditions in newborns.
Laboratory Testing Teams
Protecting Tiny Lives: The EIA Team’s Role in Newborn Screening
The Enzyme Immunoassay (EIA) Team plays a key role in the Texas Newborn Screening (NBS) Program, working tirelessly to protect the health of our youngest Texans. The EIA Team starts work early and works six days a week. With 18 dedicated staff members, the team checks newborns for four serious disorders: Galactosemia (GALT), Congenital Adrenal Hyperplasia (CAH), Congenital Hypothyroidism (CH), and Cystic Fibrosis (CF). The team uses an enzyme-linked immunosorbent assay (ELISA) method to run these tests. The assay run time can vary from three to five hours per run. The team follows different procedures for each disorder.
Disorders Screened
-
GALT: This test looks for babies who cannot process galactose, a sugar found in milk. Texas started screening for GALT in 1978. Babies with GALT cannot digest milk properly, so early detection prevents serious health issues. When the team finds abnormal screening results, they send them to the NBS DNA Analysis Team for additional screening.

-
CH: Texas added CH testing in 1980. CH occurs when the thyroid gland is not developed or does not make enough hormones, which can delay development if untreated. The EIA Team tests for CH by checking thyroid hormone levels, and early treatment helps ensure normal growth and development. Most diagnosed cases are due to developmental issues with the thyroid gland or the hormones it produces.
-
CAH: Texas began testing for CAH in 1989. Individuals with CAH do not make enough cortisol, which can cause dangerous salt imbalances in newborns. The EIA Team quickly identifies critical results to prevent life-threatening issues. They send specimens with abnormal screening results to the Mass Spectrometry Team for more testing and notify the Clinical Care Coordination (CCC) Team right away.
-
CF: Texas started screening for CF in 2009. The EIA Team checks for high levels of immunoreactive trypsinogen (IRT) in the blood, which could indicate CF. They send specimens with abnormal screening results to the NBS DNA Analysis Team for additional testing. Early treatment improves the quality of life for those with CF.
Importance of Good Specimens
Specimen quality is critical for all testing. Samples that are of poor quality, such as those caked, clotted, or layered, can lead to inaccurate results, putting newborns’ health at risk. Heat and moisture can also harm the enzymes, affecting test accuracy.
Conclusion
The EIA Team’s work is vital to keeping newborns healthy in Texas. Their efforts help detect serious disorders early, allowing for quick treatment that can greatly improve the lives of affected babies.

The NBS Metabolic Branch: Protecting Newborns by Screening 43 Conditions with One Powerful Tool!
The NBS Mass Spectrometry Team and LC Mass Spectrometry Team are vital to the Texas Newborn Screening Program. These 14 team members screen newborns for 43 conditions. They use tandem mass spectrometry to test for metabolic disorders Monday - Saturday. These include amino acid, fatty acid oxidation, and organic acid disorders.
These 14 team members screen newborns for 43 conditions. They use tandem mass spectrometry to test for metabolic disorders Monday - Saturday. These include amino acid, fatty acid oxidation, and organic acid disorders.
The tandem mass spectrometry instrument measures over 30 different biochemical markers during testing. This instrument allows the team to use one dried blood spot disk to measure more than 30 metabolic markers. They also measure additional markers by liquid chromatography- mass spectrometry to perform second-tier tests for X-linked adrenoleukodystrophy and congenital adrenal hyperplasia.
Disorders Screened:
- Amino Acid Disorders: Enzymes needed for the breakdown of specific amino acids are not produced or have reduced activity. The identification of positive screen results happens because the amino acid level is high compared to healthy babies. The team screens for disorders like Phenylketonuria (PKU) and Maple Syrup Urine Disease (MSUD). Texas has been screening for PKU since 1965 and began testing the other disorders in 2006.
- Fatty Acid Oxidation Disorders: The body cannot break down fatty acids. They are vital for the energy a baby needs to grow into a healthy toddler and live a healthy life. The best time to detect these disorders is when the baby is 24 to 48 hours old. The team screens for conditions like Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCAD) and Very Long Chain Acyl-CoA Dehydrogenase Deficiency (VLCAD). Texas started screening for these disorders in 2006.
- Organic Acid Disorders: These conditions cause a toxic buildup of organic acids. This is because the body does not have the ability to break down certain amino acids and odd-chain organic acids. The team screens for disorders like Isovaleric Acidemia (IVA) and Multiple Carboxylase Deficiency (MCD). Texas has screened for these conditions since 2006.
- X-linked Adrenoleukodystrophy (X-ALD): X-ALD is a peroxisomal and late-onset disorder. It is a condition where the body’s cells cannot break down very long-chain fatty acids due to a missing protein. The team screens all specimens for the X-ALD marker. Then, it runs a second test on some specimens to reduce false positives. Texas started screening for X-ALD in 2019.
- Congenital Adrenal Hyperplasia (CAH): After the EIA team finishes the first-tier testing, the Tandem Mass Spectrometry team runs a second-tier test to reduce false positives. This second-tier testing for CAH has been available since 2018.
Summary:
This team handles many urgent cases. They quickly report any panic-level results to the Clinical Care Coordination Team from Monday to Saturday. Some of the conditions they screen for are very rare, with only a few true positive cases in Texas over a lifetime. However, the fatty acid disorder MCAD is the most common, with about 30 cases detected each year. Overall, the team identifies around 120 infants with metabolic disorders annually. Early detection and treatment, including diet changes, help these babies and their families.

The Biotinidase Deficiency Screening Team: Pink is Our Favorite Color
Biotinidase deficiency is a condition in which the body cannot collect the vitamin called biotin from the things we eat. The body needs biotin to break down fats, proteins, and carbohydrates effectively. In 2007, Texas added screening for biotinidase deficiency to the Texas Newborn Screening (NBS) panel. From Monday through Saturday, this team of five staff members works to test all Texas babies for biotinidase deficiency.
Testing Method
 The laboratory uses liquid handling robots to test for biotinidase deficiency. The robots automatically add specific chemicals, called reagents, into the 96 wells on a plate that contains dried blood spots. These reagents get added in a specific order to create a color changing reaction. Once the color forms, the team quickly checks the plates to see the results. If the specimen turns pink, it means that biotin is being collected, and the screening test result is normal. If the specimen stays straw-colored or colorless it means biotin may not be present. This means the baby may have biotinidase deficiency and cannot collect biotin properly.
The laboratory uses liquid handling robots to test for biotinidase deficiency. The robots automatically add specific chemicals, called reagents, into the 96 wells on a plate that contains dried blood spots. These reagents get added in a specific order to create a color changing reaction. Once the color forms, the team quickly checks the plates to see the results. If the specimen turns pink, it means that biotin is being collected, and the screening test result is normal. If the specimen stays straw-colored or colorless it means biotin may not be present. This means the baby may have biotinidase deficiency and cannot collect biotin properly.
Challenges Texas Weather Poses to These Specimens
Excessive heat or humidity affects biotinidase and can impact the test result for biotinidase deficiency. Therefore, we see an increase in positive cases during the summer months, especially if the NBS specimens have been sitting outside in a mailbox. We encourage providers to ship via courier when possible and to limit exposure of NBS specimens to heat and humidity.
Conclusion
Children with biotinidase deficiency need extra biotin, or health problems usually result. Texas identifies about four to six infants annually with biotinidase deficiency. If not treated, a baby can have seizures, hearing loss, vision problems, and death in severe cases. This condition is treatable, and treatment involves a daily dose of vitamin biotin.

The Hemoglobinopathy Team: Banding Together
Texas started testing for hemoglobinopathies in 1983. Hemoglobinopathies are genetic disorders. They affect hemoglobin, a protein in red blood cells that carries oxygen. The Hemoglobinopathy Team is a group of ten staff members that work Monday through Saturday.
 Disorders Screened
Disorders Screened
-
Sickle cell disease is the most well-known hemoglobinopathy in Texas. Sickle cell disease affects the hemoglobin within red blood cells. A genetic mutation causes abnormal hemoglobin to clump together, causing the red blood cells to become sickle shaped. These sickle-shaped cells cause blockages in your blood flow, which can lead to anemia, blood clots, chronic pain, infections, stroke, and severe complications. Management of sickle cell disease is lifelong. Treatment may include pain management, limitations on exercise, and blood transfusions. Early detection of sickle cell disease is important. Babies born with sickle cell disease are more prone to serious infections and may need antibiotics.
-
People who have sickle cell trait inherit a hemoglobin S gene from one parent and a normal gene from their other parent. Most people with sickle cell trait do not have any symptoms of sickle cell disease.
-
Other hemoglobin variants and types of thalassemia may also be detected.
Testing Methods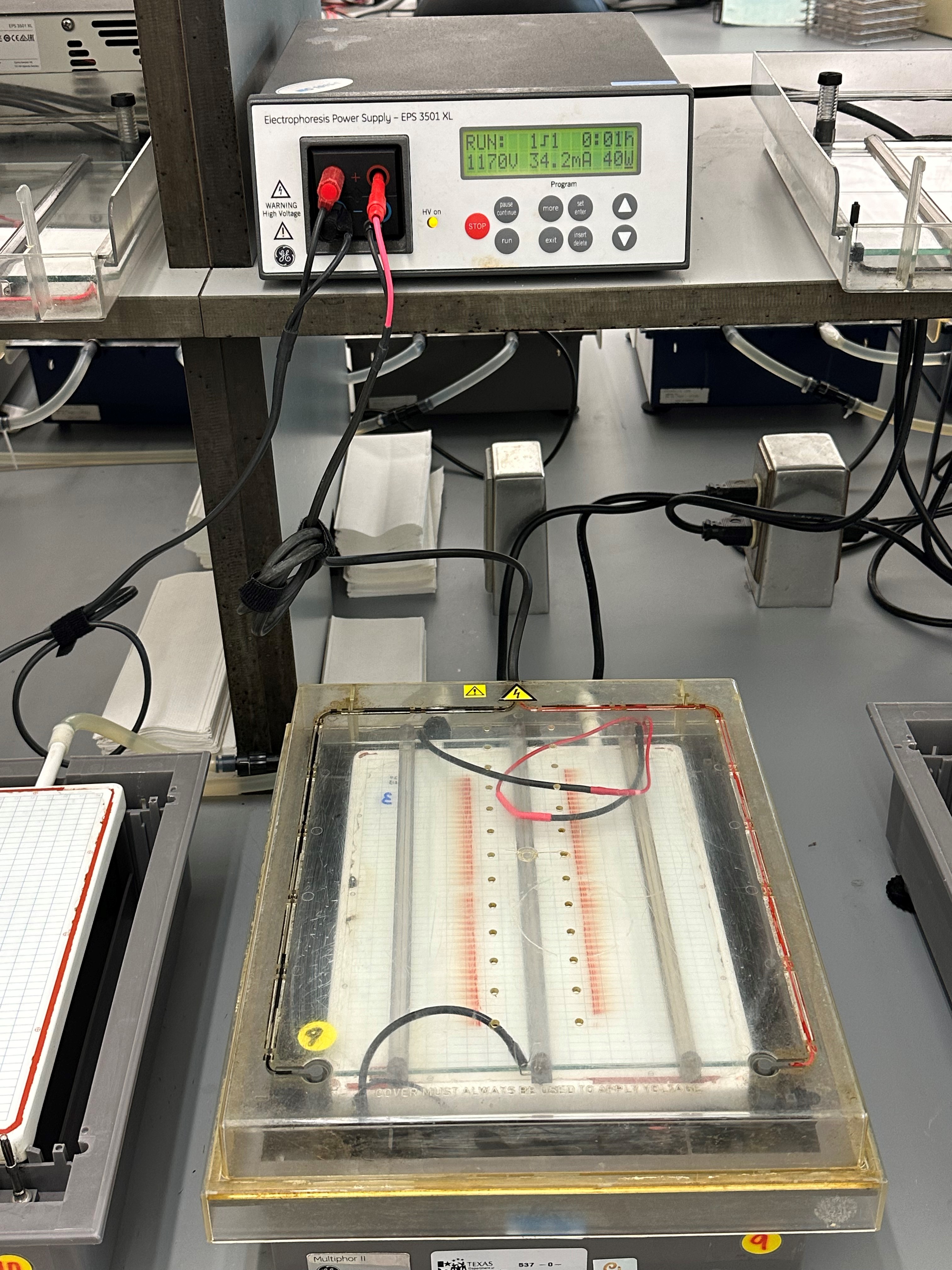
The team runs an agarose gel using isoelectric focusing to separate the hemoglobin proteins on the gel. Using electrical current, the hemoglobin moves through the gel, leaving visible bands. Staff review each banding pattern on the gels using a manual review process with light boxes. By looking at the gels, they can identify various types of hemoglobins. The team can identify fetal hemoglobin (Hb F), adult hemoglobin (Hb A), other hemoglobin, and those that are missing hemoglobin.
When abnormal hemoglobins are found, secondary testing using high performance liquid chromatography (HPLC) can confirm the results. This test produces a chromatogram. This chromatogram shows when and how long each hemoglobin takes to pass through a column.
 Importance of the Demographic Information
Importance of the Demographic Information
Transfusion status is important when the team screens for hemoglobinopathies. The newborn screening demographic form has a space to record transfusion status. When a baby gets transfused, the trait status of the donor can interfere with the test results. Interference can also occur with intrauterine transfusions.
Follow-up
The Clinical Care Coordination Team coordinates follow-up guidance for clinically significant hemoglobinopathies. They also send notification letters to the families of babies who have the sickle cell trait.
Conclusion
With hemoglobinopathies, early treatment means a better outcome for the baby. On average, Texas has about 10 sickle cell disease newborns born in Texas per month. Texas reports about 500 babies a month with sickle cell trait.
The Severe Combined Immunodeficiency (SCID) and Spinal Muscular Atrophy (SMA) Team: Excitation and Emission
The SCID/SMA team consists of 12 individuals who work Monday through Saturday. This team runs a single test that looks for both disorders at the same time.
Disorders Screened
-
Texas has been screening for Severe Combined Immunodeficiency (SCID) since 2012. SCID is a group of genetic disorders that affect the development of the immune system.
-
In 2021, Texas added Spinal Muscular Atrophy (SMA) to the newborn screening panel. SMA is a progressive neurodegenerative disease that affects the motor nerve cells of the spinal cord. Defects in the SMN1 gene cause SMA. SMA is the number one genetic cause of death for infants.
Testing Method
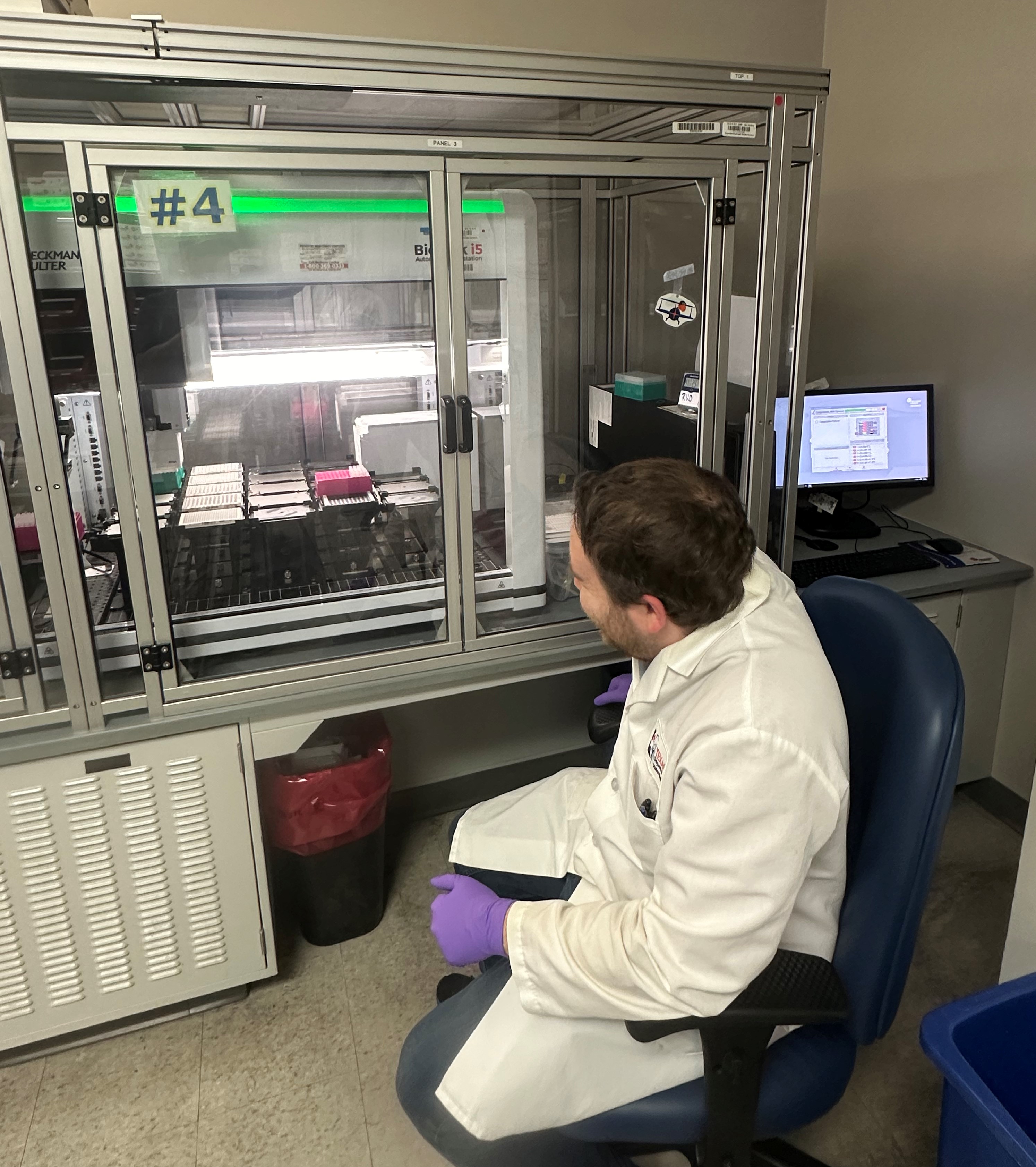 The team loads the 96-well plates, containing the dried blood spots, onto a robotic liquid handling system, which washes the dried blood spots and extracts the DNA using a DNA extraction buffer. After adding the buffer, the team seals the plates and places them on a thermal cycler to heat the DNA extract. They then use the same robots to pipette a small amount of the extracted DNA from the original plate into a new plate containing the test reagents. The team places this plate on a real-time polymerase chain reaction (PCR) instrument that detects the target DNA sequences using fluorescent molecules. The instrument shines light at the sample, causing the molecules to fluoresce (excitation and emission) and then measures the fluorescence after every PCR cycle. In the SCID-SMA test, the instrument detects part of the SMN1 gene for SMA and T-cell receptor excision circles (TREC) for SCID. TREC is an indicator of T-cell maturity. If TREC is not present, it could indicate SCID.
The team loads the 96-well plates, containing the dried blood spots, onto a robotic liquid handling system, which washes the dried blood spots and extracts the DNA using a DNA extraction buffer. After adding the buffer, the team seals the plates and places them on a thermal cycler to heat the DNA extract. They then use the same robots to pipette a small amount of the extracted DNA from the original plate into a new plate containing the test reagents. The team places this plate on a real-time polymerase chain reaction (PCR) instrument that detects the target DNA sequences using fluorescent molecules. The instrument shines light at the sample, causing the molecules to fluoresce (excitation and emission) and then measures the fluorescence after every PCR cycle. In the SCID-SMA test, the instrument detects part of the SMN1 gene for SMA and T-cell receptor excision circles (TREC) for SCID. TREC is an indicator of T-cell maturity. If TREC is not present, it could indicate SCID.
Follow-up on Abnormal Results
If a specimen screens abnormally and at a panic level for SCID, the team will notify the Clinical Care Coordination Team right away for follow-up.
Results that screen abnormal for SMA get sent on to the NBS DNA Analysis Team for more testing. SMA is time-critical. The Clinical Care Coordination Team takes immediate action when there is an abnormal screen.
Importance of the Demographic Information
The newborn screening demographic form has a space to record transfusion status. Transfusion status is important when we screen for SCID. The test looks for T-cell receptor excision circles (TREC), which indicate a functioning immune system. Babies without a functioning immune system will have very low levels of TREC compared to healthy babies. In adults, TREC levels are naturally low due to an already mature immune system. A transfusion can artificially lower a baby’s TREC levels, as transfused blood lacks TREC, potentially leading to an abnormal result.
Conclusion
The SCID/SMA Team’s work plays an important part in keeping the newborns of Texas healthy. They screen babies for these disorders because treatment is available and effective.
Behind the Screens: How the Newborn DNA Analysis Team Confirms Screening Results
The Newborn DNA Analysis Team consists of 13 dedicated members. They perform molecular confirmatory testing for specific abnormal newborn screening (NBS) results. Verifying initial findings and providing accurate information to healthcare providers is critical.
Disorders Screened and Methods Used: 

The team handles confirmatory screening tests for several disorders using various methods. DNA testing improves the value and reduces the chance of false positive initial screens. Different test methods can identify sequence variants that cause a particular disorder.
- Cystic Fibrosis (CF): They use a 60-variant panel for testing.
- Galactosemia: This involves polymerase chain reaction (PCR) and gel electrophoresis.
- Hemoglobinopathies: They perform allelic discrimination using real-time PCR and Sanger sequencing.
- Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCAD): They perform allelic discrimination using real-time PCR.
- Spinal Muscular Atrophy (SMA): They quantify copy number variation using digital droplet PCR.
- Very Long Chain Acyl-CoA Dehydrogenase Deficiency (VLCAD): Sanger sequencing is the method used.
- X-Linked Adrenoleukodystrophy (XALD): Sanger sequencing is also used for this disorder.
The team evaluates the pathogenicity of variants according to the American College of Medical Genetics and Genomics (ACMG) guidelines and current research. All information is for screening purposes and not diagnostic.
The initial NBS result report includes DNA results for disorders like CF and SMA. Some sequencing results can take up to two weeks to process. A second report will contain the results of the extended time tests.
can take up to two weeks to process. A second report will contain the results of the extended time tests.
The team’s work ensures that healthcare providers have as much information as possible to provide appropriate care for the baby.

Essential Support Teams
Driving Efficiency and Communication: The Role of Newborn Screening Administrative Assistants
Newborn Screening (NBS) Administrative Assistants are at the core of the NBS Program. They manage communication between NBS teams and external stakeholders. The oversight ensures a smooth and successful operation. By handling calls, emails, and other messages, they ensure inquiries go to the right person. This keeps information flowing and resolves issues quickly. It's crucial for timely action that affects newborn health outcomes.
The Administrative Assistants also organize meetings, training, and events. They coordinate schedules, prepare materials, and ensure all resources are ready. Their planning and organization keep the program on track. They enable effective discussions and decisions.
Newborn Screening Administrative Assistants provide vital support to the NBS Program, allowing the program to focus on the mission: to detect and intervene early to protect newborns' health.
Empowering Providers and Families: The Role of the Newborn Screening Educational Team
The Newborn Screening (NBS) Education Team promotes NBS across the state. The team educates healthcare providers, hospitals, birthing centers, and families of newborns. Educating about the importance of NBS is the top priority.
The team creates educational materials including brochures, online resources, and custom training sessions. A few of the topics covered are:
- Completing the Demographic Form
- Storage and Use of Newborn Screening Blood Spots
- Quality Specimen Collection
- Timely Specimen Submission
The Education Team works with other NBS teams in the DSHS Lab on quality improvement projects, such as analyzing data and targeted outreach efforts to improve the NBS process in Texas. Submitters and families can always contact the NBS Education Team for help. Sharing up-to-date information empowers submitters and families.

Innovating and Optimizing Newborn Screening Through Informatics

Newborn Screen Laboratory Informatics Team
Newborn screening (NBS) requires fast and effective information sharing between organizations. The NBS Laboratory Informatics Team dedicates itself to enhancing the efficiency and accuracy of the newborn screening process using advanced technological solutions. The team of two specialists and one manager form a partnership with the main goal to support the Laboratory Information Management Systems (LIMS) within the Newborn Screening Laboratory.
Key Responsibilities:
-
Project Management and LIMS Change Requests:
-
The team manages various projects and handles change requests aimed at enhancing LIMS. This includes implementing new features, resolving existing issues, and adding new disorders to the screening capabilities.
-
-
Interoperability:
-
The team facilitates the integration of the HL7 interface, ensuring seamless communication between external partners and the NBS laboratory for newborn screening data.
-
-
Issue Resolution and Troubleshooting:
-
A critical team function is to troubleshoot and resolve any issues within the LIMS. This ensures minimal disruption and maintains the accuracy and reliability of the screening process.
-
-
Data Analytics:
-
The team performs data analytics and creates visualizations to track trends in testing quality measures and to support the business activities of the newborn screening program. This helps identify issues early and improve the screening process.
-
By focusing on these areas, the NBS Laboratory Informatics Team plays a vital role in advancing public health through timely and accurate newborn screening.
Maintaining Standards: The Quality Assurance Officer’s Role in Newborn Screening
The Newborn Screening (NBS) Laboratory has a dedicated Quality Assurance (QA) Officer. The QA Officer’s oversight ensures we meet the certification requirements of both the Clinical Laboratory Improvement Amendments (CLIA) and the College of American Pathologists (CAP). The QA Officer keeps the laboratory on track with clear procedures and guidelines, including regular meetings, internal audits, and corrective actions to address any issues. The QA Officer also manages training programs to keep the staff current on quality policies and regulatory requirements. Their work ensures that newborn screening tests are accurate and reliable; another important component to protecting the health of newborns across the state.

Laboratory Reporting Team – Customer Service with a Technical Twist
The Laboratory Reporting Team dedicates itself to timely and accurate entry of specimen demographic data and timely reporting of Lab results. The team of 29 members support three areas of the DSHS Laboratory: newborn screening (NBS), Texas Health Steps, and the Microbiology Program. The team assists submitters with getting their patient’s results without delay.
Key Responsibilities:
- Processing Specimen Demographic Forms:

- This year the team processed over 760,000 NBS specimen demographic forms. Handwriting is very important to the work this team does. Writing in block capital letters helps the team to interpret and accurately enter the information.
- The team also enters demographic forms for Texas Health Steps and Microbiology specimens received for testing.
- Lab Reporting enters over one million specimen demographics each year across all three programs.
- Performs Quality Checks on Key Data Elements:
- The team performs daily quality checks on required data elements. They generate daily reports and track workflows to keep the process moving.
- Enroll Submitters for DSHS Testing Services:
- The team maintains the submitter accounts at the DSHS Laboratory. They enroll new submitters for DSHS testing and update submitter information as requested. It’s important that updated information be on file for each submitter location.
- Customer Service
- Each year the team answers more than 35,000 submitter telephone calls. The team provides submitters with testing results and answering general questions. The team serves as a main point of contact for the DSHS Laboratory.

Sending Result Reports to Submitters:
- A critical function of the team is to send out lab testing results daily. Submitters receive results based on their selected preference: mailed, faxed, HL7 or NBS online portal. Lab Reporting sends out over one million reports yearly.
- We encourage all submitters to have an NBS online portal account. Having an online account allows self-service for accessing NBS results. Each year this team receives and processes over 95,000 requests for duplicate screening results.
- Archiving Demographic Forms:
- The team ensures that demographic forms are securely stored. The State Library stores the forms for a set amount of time for future reference or until destroyed.
This team plays a critical role in keeping the NBS testing process moving. They are always available to aid submitters with obtaining NBS results.

Innovator in Action: How Research Specialists Are Shaping the Future of Newborn Screening
A Research Specialist in the Medical Screening Unit is key to advancing how we detect rare diseases in newborns. They develop and confirm new testing methods. The work ensures the laboratory stays at the cutting edge of technology. The Research Specialist keeps detailed records of the laboratory's technologies. They also identify improvements for the future. The Specialist collects and analyzes research on newborn screening disorders. Their work is essential for expanding and improving newborn screening in Texas.
DSHS Newborn Screening Clinical Care Coordination
Texas Early Hearing Detection and Intervention Team
The Newborn Screening (NBS) Texas Early Hearing Detection and Intervention (TEHDI) Team provides statewide newborn hearing screening surveillance and supports early intervention services for babies diagnosed as deaf or hard of hearing (D/HH).  The team promotes education and resources to families and healthcare providers, providing guidance and follow-up care coordination as needed. The team understands crucial timelines and steps to manage hearing loss, as it impacts children’s development and well-being.
The team promotes education and resources to families and healthcare providers, providing guidance and follow-up care coordination as needed. The team understands crucial timelines and steps to manage hearing loss, as it impacts children’s development and well-being.
Hearing loss is the second most common birth defect in Texas. Texas identifies approximately 500 babies with hearing differences each year.
Key Responsibilities:
Promoting Joint Committee on Infant Hearing (JCIH) 1-3-6 Guidelines:
- The JCIH 1-3-6 guidelines provide a timeline for newborn hearing screening and diagnosis;
- Hearing screening by one month of age;
- Diagnostic hearing evaluation by three months; and
- Enrollment in early intervention by six months.
- Timely intervention is key for infants with hearing loss. Hearing is crucial for language, social, and cognitive development. Early action can prevent potential developmental delays.
TEHDI Data Reporting:
- The TEHDI Management Information System (MIS) records hearing screening services for Texas babies.
- Health care providers who perform hearing screening services must report all patient encounters in the TEHDI MIS. Encounters include the initial hearing screen, follow-up screening, diagnostic evaluations, and follow-up care, or interventions. Reporting includes “pass” and “do not pass” result outcomes. Providers must enter the information within five calendar days of the patient encounter. The team compiles an annual data report to the Centers for Disease Control and Prevention (CDC); and
- TEHDI also provides MIS technical assistance.
Certifying newborn hearing screening programs:
- TEHDI certifies newborn hearing screening programs across the state.
- Regular certifications demonstrate newborn hearing screening programs meet basic newborn hearing screening requirements.
Partnerships
TEHDI partners with the Statewide Outreach Center at Texas School of the Deaf (SOC at TSD). Through this partnership, we support families and babies identified as D/HH through age 21.
TEHDI also connects with Texas Hands and Voices, a parent-to-parent family-based organization. Together they help strengthen local support and build a comprehensive referral system.
To learn more about the TEHDI, visit DSHS Texas Early Hearing Detection Intervention TEHDI.
Newborn Screening Benefits Team
The Newborn Screening (NBS) Benefits Team provides Program guidance to contracted vendors. The Team also processes and maintains client enrollment data. The NBS Benefits Program serves eligible Texans that have one of the disorders screened for by NBS Benefits include dietary supplements, medications, testing and follow-up care.

The NBS Benefits Team has two Program Specialists providing services to over 200 individuals each year. This Program stands out with its customized approach and goal of serving those most in need with no other source of coverage. A family’s income must be at or below the 350% Federal Poverty Income Limit. Families cannot be on other forms of assistance, such as Medicaid. The Program’s partnerships with hospitals and clinics helps promote services to those most in need.

 Team’s Key Responsibilities:
Team’s Key Responsibilities:
- Processing benefit requests;
- Managing all applications;
- Working with the Newborn Screening Medical Director on eligibility; and
- Working with contracted partners who provide benefits to those who qualify.
To learn more about the NBS Benefits Program, visit DSHS Newborn Screening Benefits Program
 Special Projects Team
Special Projects Team
The Special Projects team sprang into existence in November of 2023. This was due to unit growth and the ever-expanding Texas newborn screening (NBS) panel. The team supports the entire NBS Unit. They manage and coordinate many projects such as: grant activities, contracts, the Texas NBS Education Conference, the Genetics Medical Student Internship Program, continuing education, records retention, advisory committee coordination, Texas Administrative Code updates, and so much more.
Team
Each team member has key responsibilities.
- Manager – Oversees the special project team and acts as a stand-in for the Unit Director when needed;
- Unit Coordinator – Serves as a contact for general admin operations. Provides guidance to NBS programs. Coordinates assignment responses and approvals. Oversees legislative processes affecting NBS programs and facilitates assignments from analysis to rule drafting to final implementation. Works on special projects as assigned and assists with advisory committee coordination;
- Ombudsman and Advisory Committee Liaison – Supports and coordinates the Newborn Screening Advisory Committee and the Sickle Cell Task Force. Addresses complaints about the NBS Program. Assists with legislative activities as needed;
- Data Informaticist – Retrieves and interprets unit data to create clear visuals and reports about its activities;
- Educator – Develops and presents NBS education to staff, government agencies, medical schools, community health organizations, and the public. Serves as a primary contact for these entities;
- Website and Communications Specialist – Maintains the NBS Clinical Care Coordination website. Helps update and create educational materials, such as brochures about NBS and screened conditions; and
- Grant Coordinator – Maintains NBS grants and serves as lead contact and facilitator for grant-related activities impacting the NBS Unit.
The members of this team often help with tasks outside their key roles. This means team members are familiar with the many aspects of NBS. Having a well-versed staff is a huge benefit to the Unit.
Critical Congenital Heart Disease (CCHD)
CCHD screening is a point-of-service test to determine if an infant has a serious heart condition. The birthing facility will conduct the screening by measuring how much oxygen is in the baby’s blood. The screening test only takes a few minutes. Babies with low blood oxygen levels will have immediate access to diagnostic testing and care. Infants with CCHD usually need surgery. State law requires providers to report confirmed CCHD cases to the Texas Department of State Health Services (DSHS).
The NBS Special Projects Team coordinates Texas CCHD activities including:
- The Unit Coordinator facilitates legislative assignments, including any proposals to change statute.
- The NBS Advisory Committee Liaison supports the Texas Pulse Oximetry Project CCHD toolkit administration. This toolkit provides educational and technical information on screening for CCHD. The toolkit is available at DSHS Newborn Screening Program – CCHD in Texas; and
- The Data Informaticist organizes the CCHD confirmed cases information received and compiles reports.
To learn more about CCHD Screening in Texas, visit DSHS Newborn Screening Program – CCHD.
DSHS Newborn Screening Clinical Care Coordination
The Department of State Health Services (DSHS) Newborn Screening (NBS) Unit has a team of 63 members. The number of staff is robust, but the volume of work is greater. Clinical Care Coordination (CCC) staff work six days a week, including holidays, protecting Texas babies. The NBS Unit Director leads the work of the Unit, and the Medical Director oversees all clinical matters. Together, they work towards the goal of ensuring all babies born in Texas receive appropriate NBS follow-up care.
The number of staff is robust, but the volume of work is greater. Clinical Care Coordination (CCC) staff work six days a week, including holidays, protecting Texas babies. The NBS Unit Director leads the work of the Unit, and the Medical Director oversees all clinical matters. Together, they work towards the goal of ensuring all babies born in Texas receive appropriate NBS follow-up care.
There are four CCC Follow-Up Teams with a focus on the following disorder types:
- Metabolic disorders;
- Thyroid disorders;
- Hemoglobin disorders; and
- Congenital Adrenal Hyperplasia.

The CCC Follow-Up Teams focus on coordinating follow-up care for out-of-range screening tests. Registered nurses, public health and prevention specialists, and a team manager make up each team. The teams initiate over 1,900 follow-up protocols each year. Their work involves communicating next steps with medical providers and families. The team will follow each case until the baby receives a diagnosis or cleared status. They all work together to carry out key responsibilities.
Key Responsibilities:
- Deliver out-of-range screening results;
- Provides the infant’s primary care provider or other healthcare provider the NBS results and next steps;
- Discuss recommendations and resources to health care providers and families; and
- Coordinates with providers and specialists for confirmatory testing or treatment.

Long-Term Follow-Up:
- The CCC Follow-Up Team includes a Long-Term Follow-Up Specialist. All diagnosed cases receive long-term follow-up, except for Sickle Cell Trait and Critical Congenital Heart Disease. Follow-up occurs at varying times in the first year of life depending on the disorder diagnosed. Each diagnosed case receives annual follow-up after the first year of life.

To learn more about the Clinical Care Coordination Team visit DSHS Newborn Screening Program. To learn more about the disorders on the screening panel, visit DSHS Newborn Screening – Newborn Screening Disorders.

Lab Technician in Specimen Processing

Laboratory Technician Running an Instrument

DNA Staff Member holding a Erlenmeyer flask (Beaker)

Laboratory Technician
All Photos taken by DSHS Public Health Laboratorian Staff
Book traversal links for We Are Newborn Screening
- We Are Newborn Screening
- DNA Analysis Laboratory
- Newborn Screening Notices
- Newborn Screening - Use and Storage of Dried Blood Spots after NBS
- Newborn Screening - Use of NBS Blood Spots after Completion of Newborn Screening
- Newborn Screening Parent Resources
- NBS Annual Report
- Newborn Screening - Frequently Asked Questions

