Using the DSHS IRB OneAegis System
The DSHS IRB OneAegis System is an online platform to submit DSHS IRB applications. Access to the System is obtained by registering with IAMOnline.
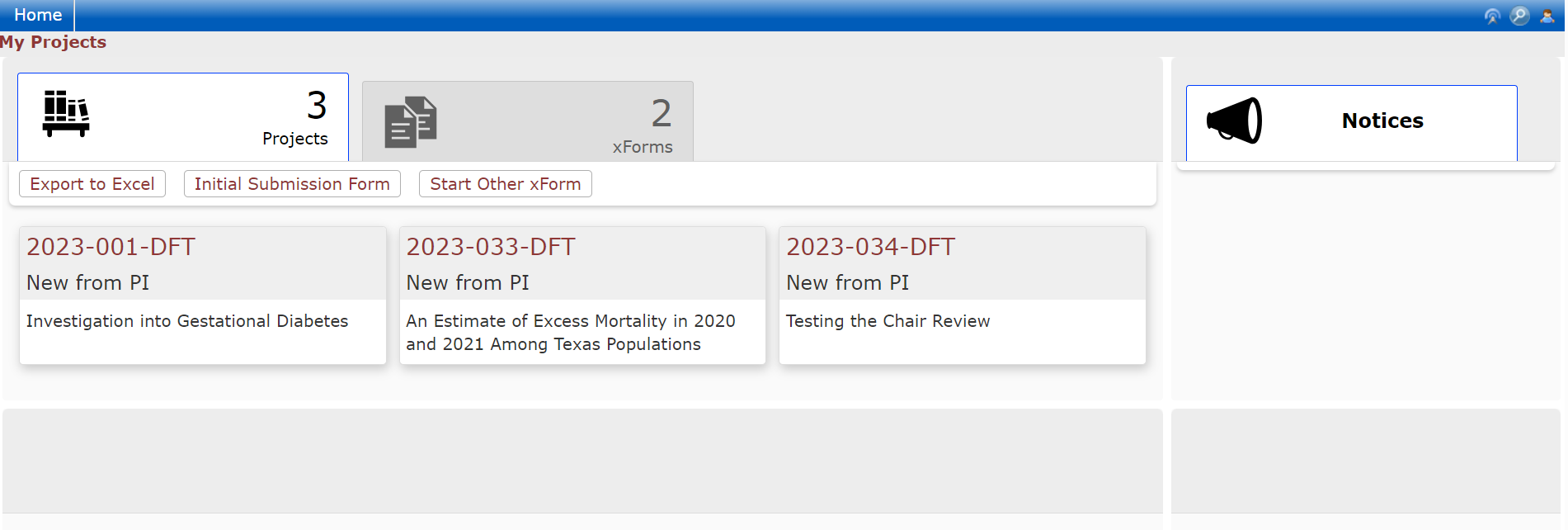
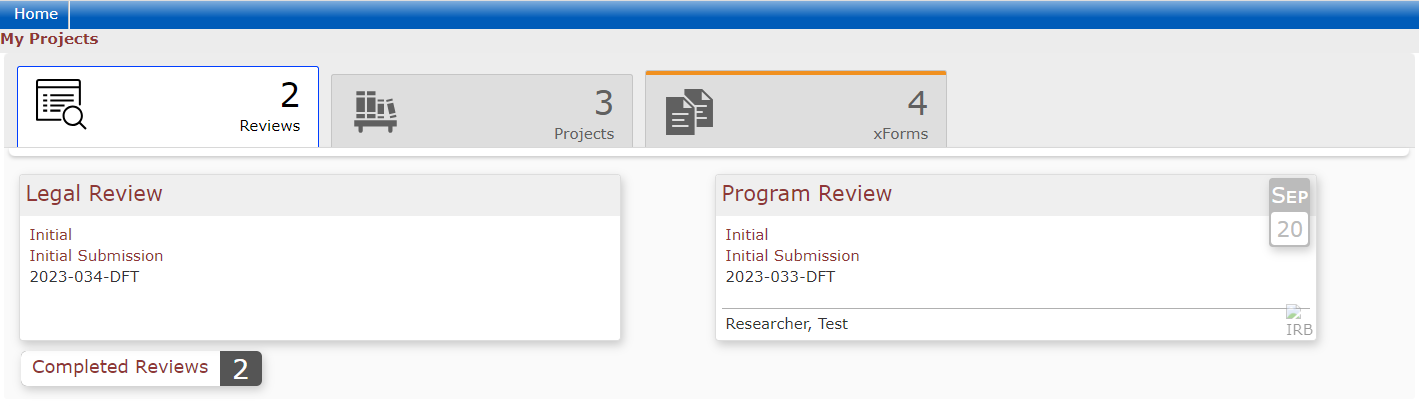
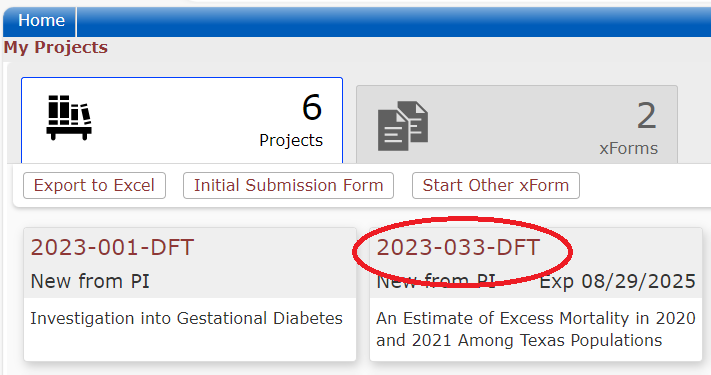
The Dashboard
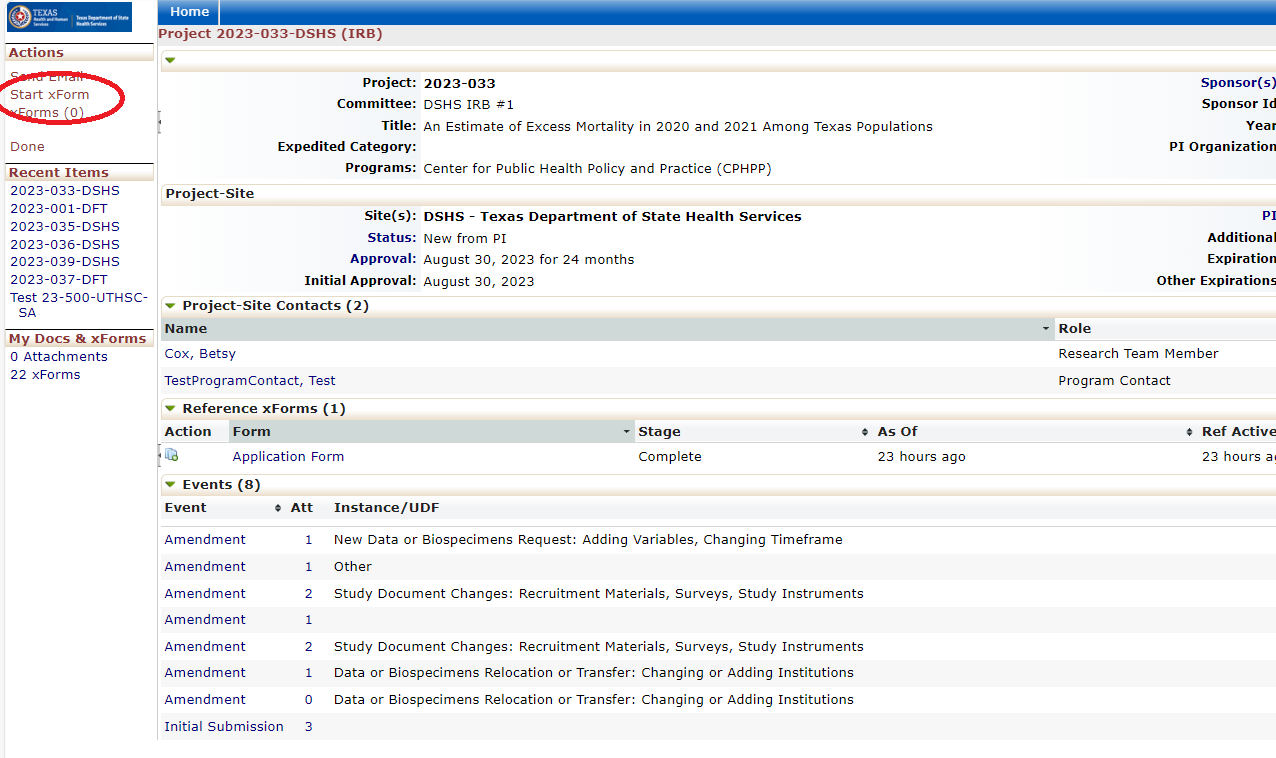
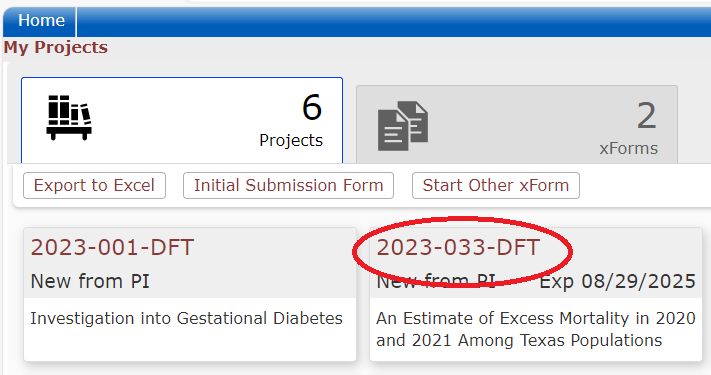
When users log into the DSHS IRB OneAegis site they will be presented with a dashboard with information on their applications or applications to review.
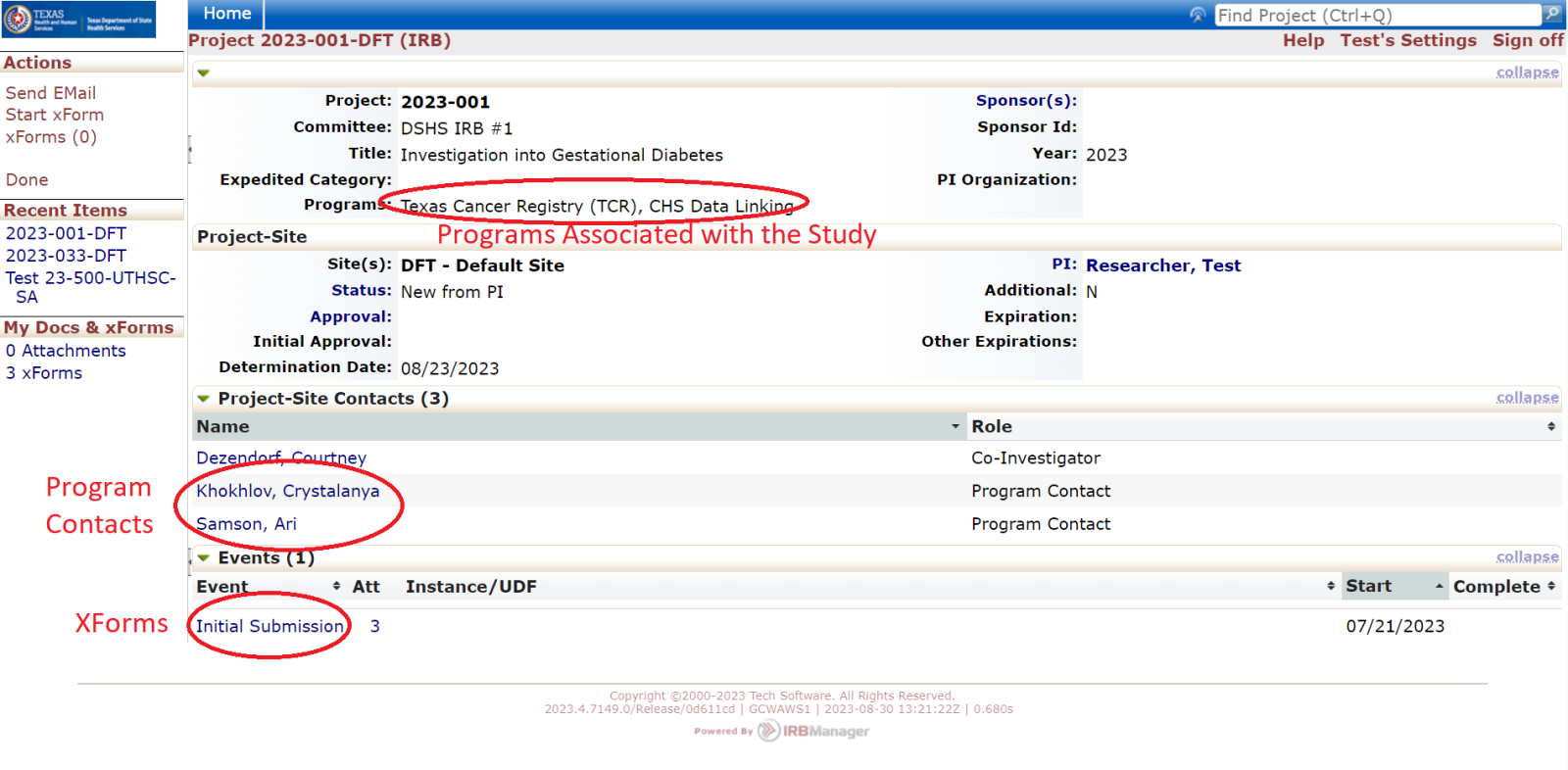
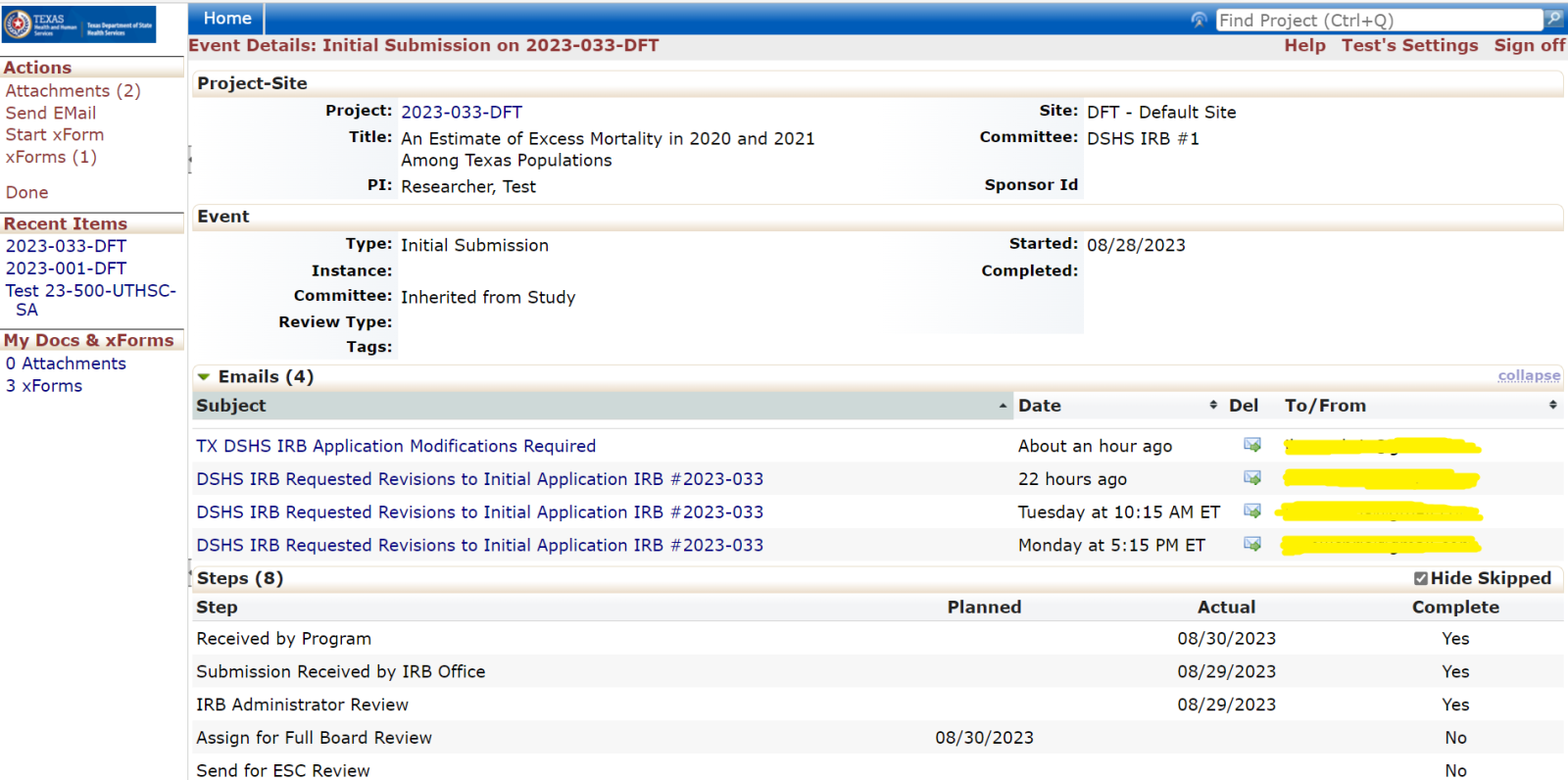
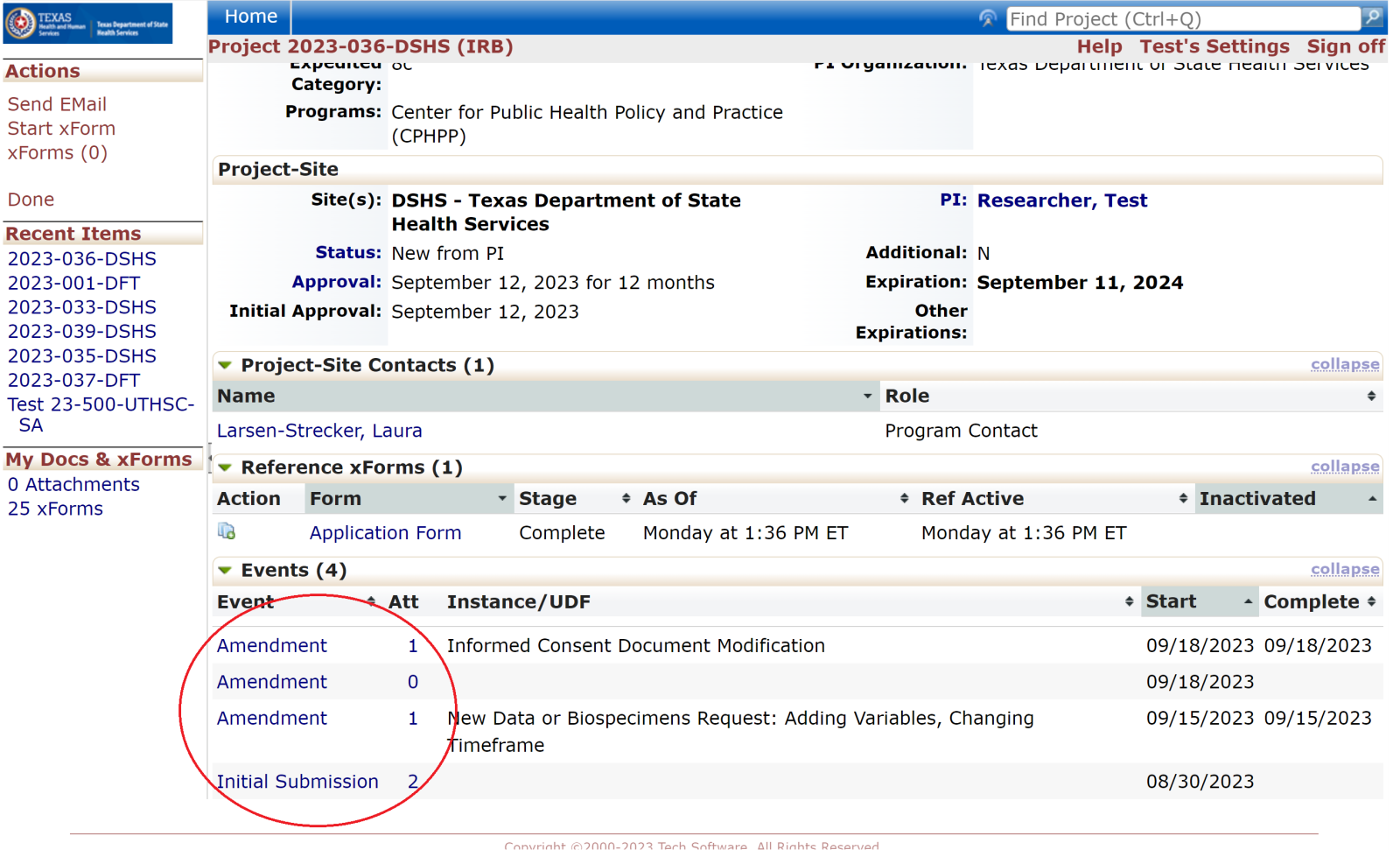
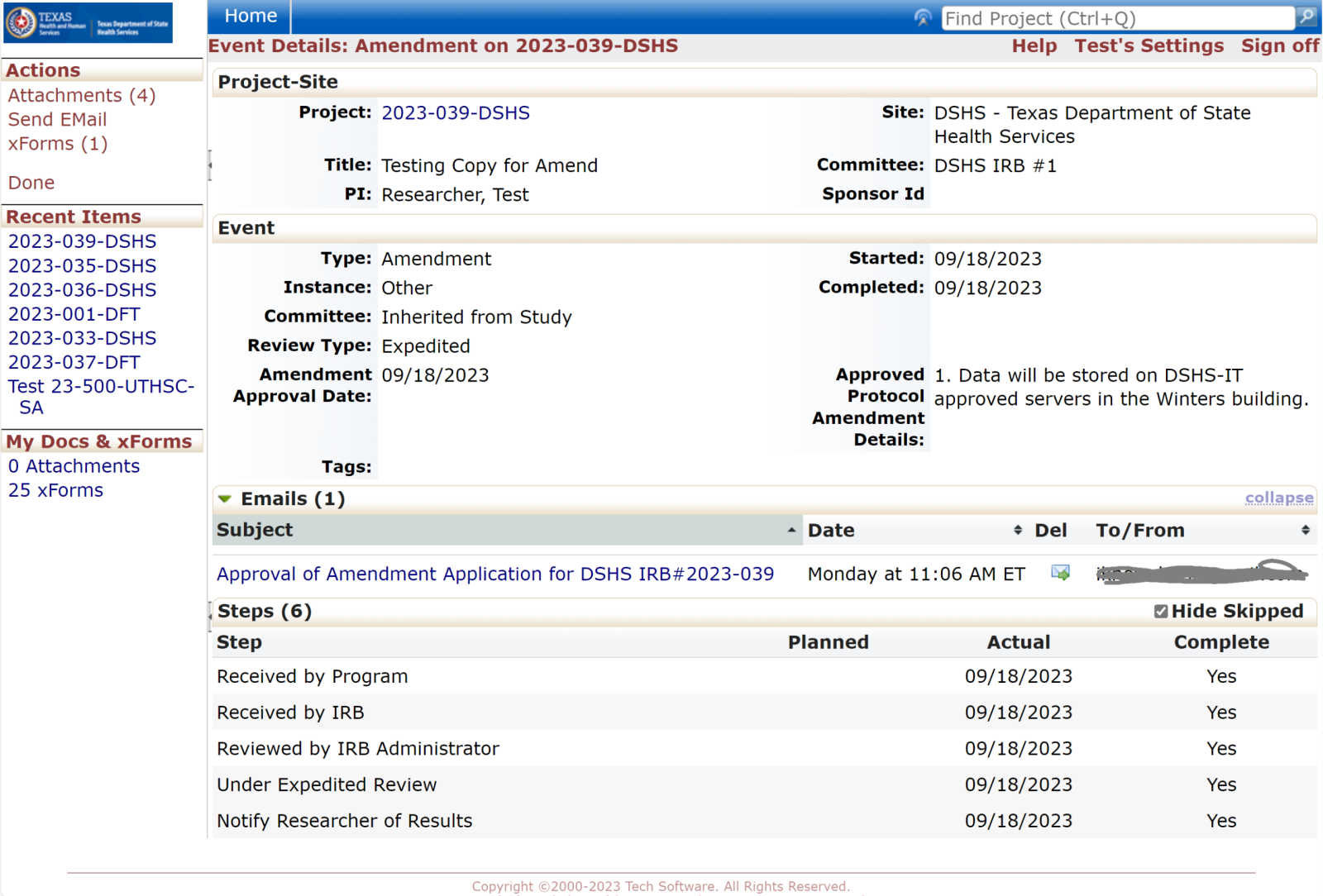
Events
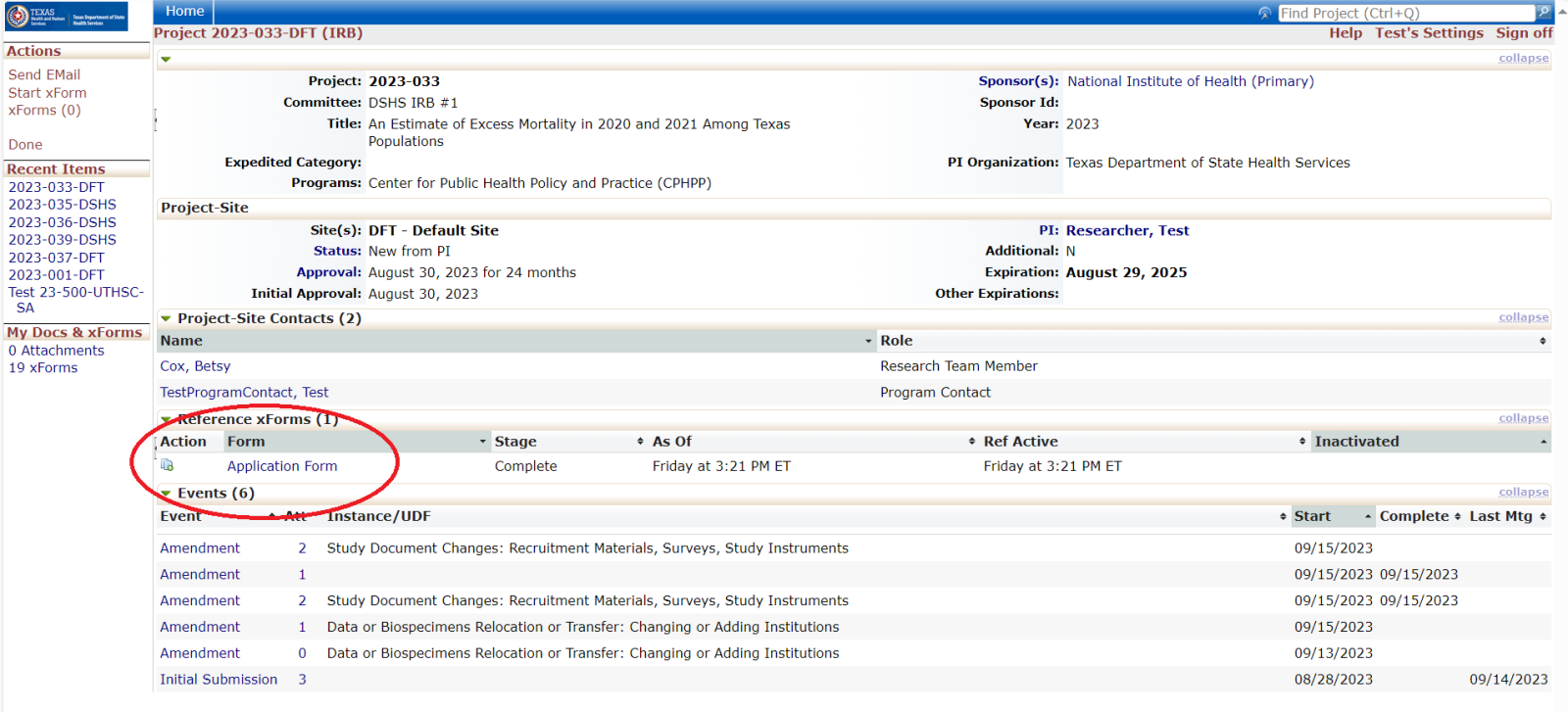
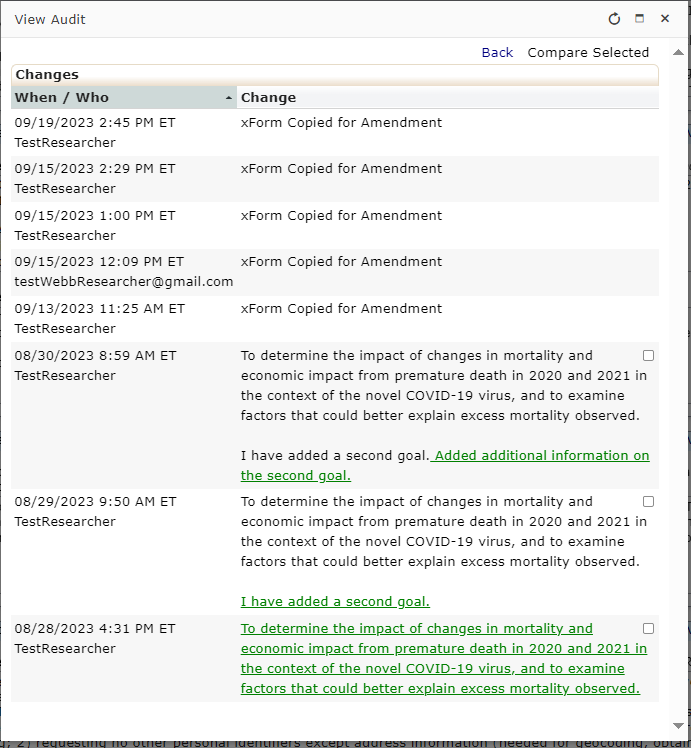
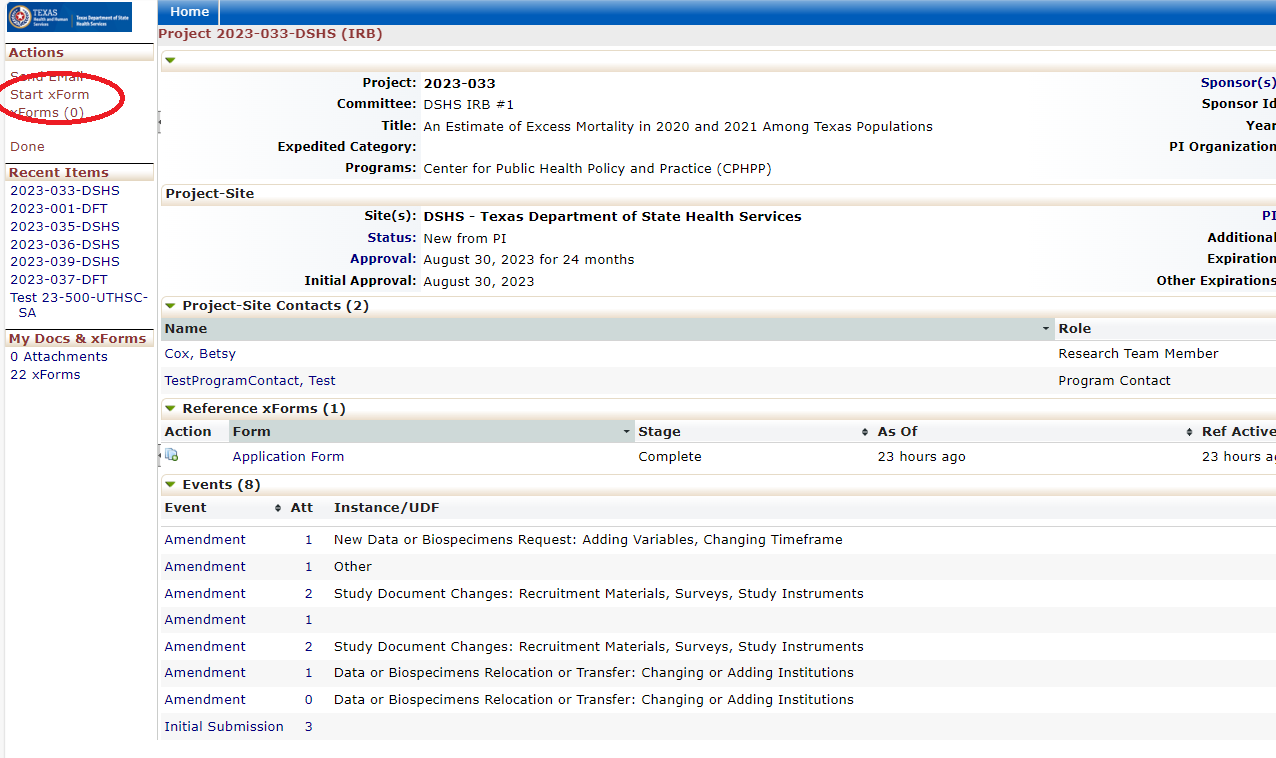
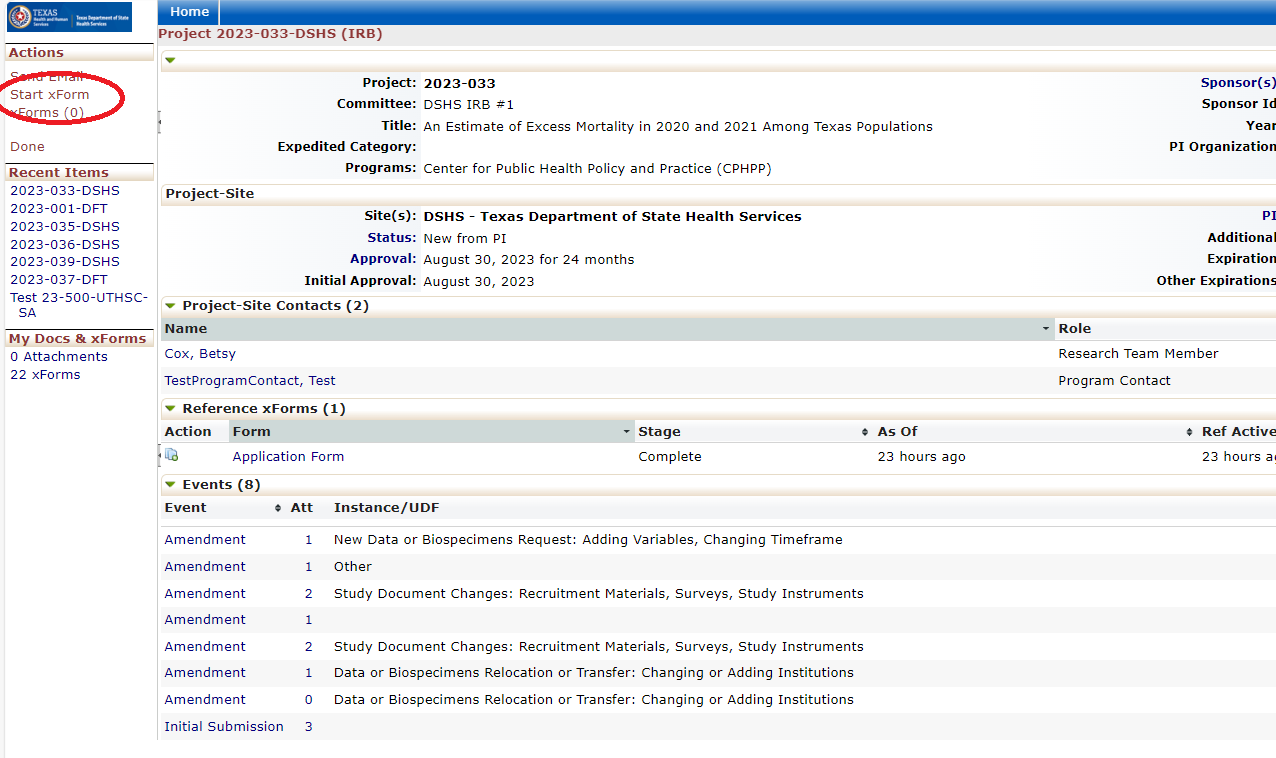
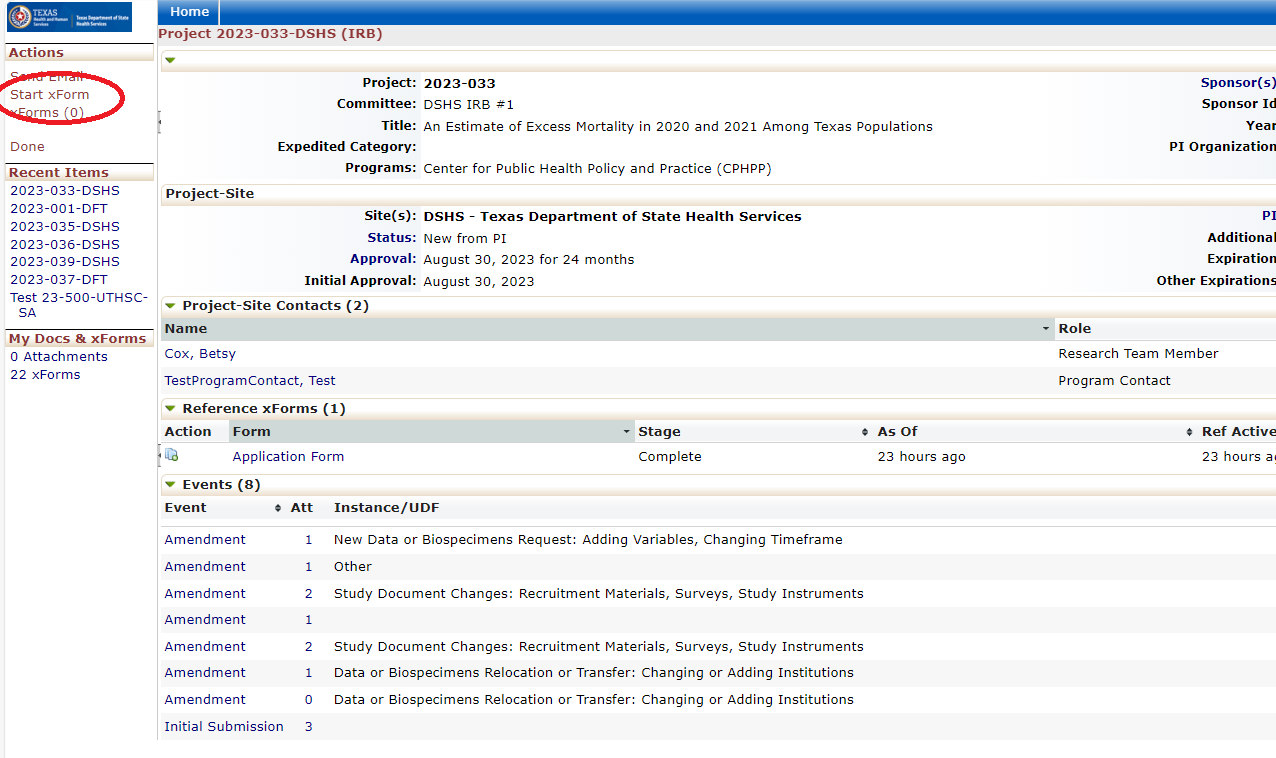
Every review or submission in the DSHS OneAegis IRB system is an event. Researchers have access to protocols and all event information in the IRB system for protocols that are assigned to them.
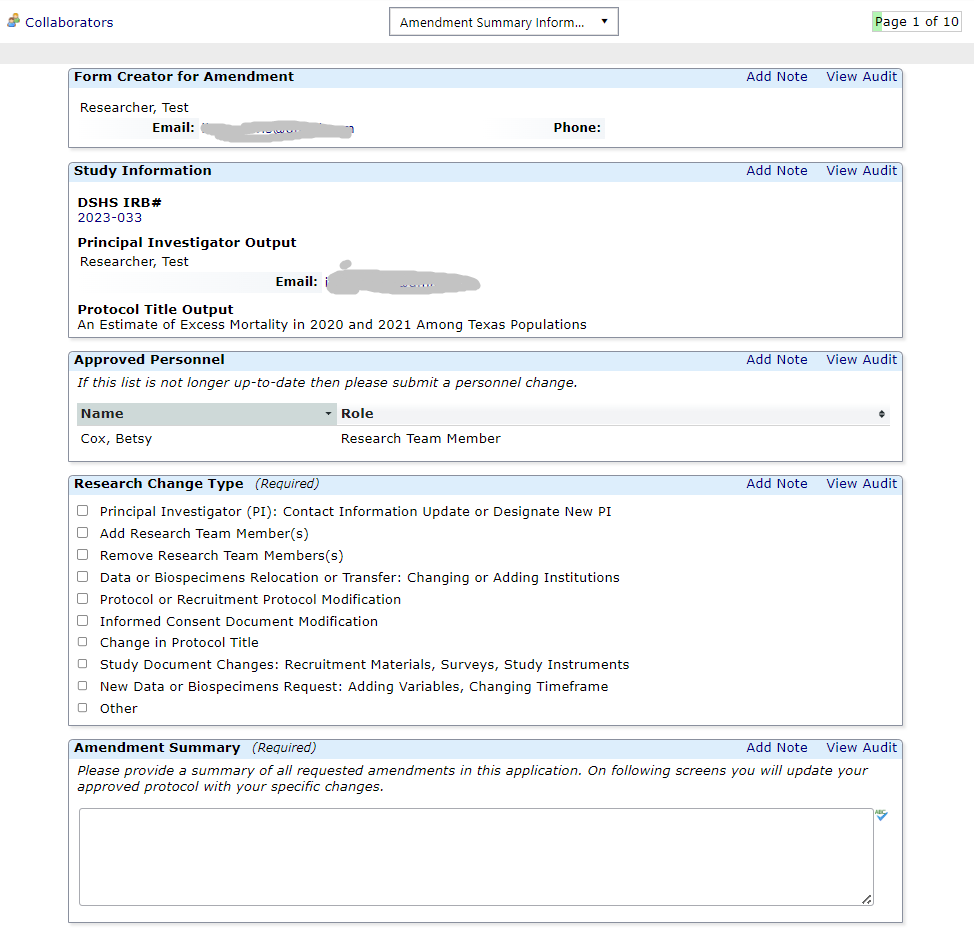
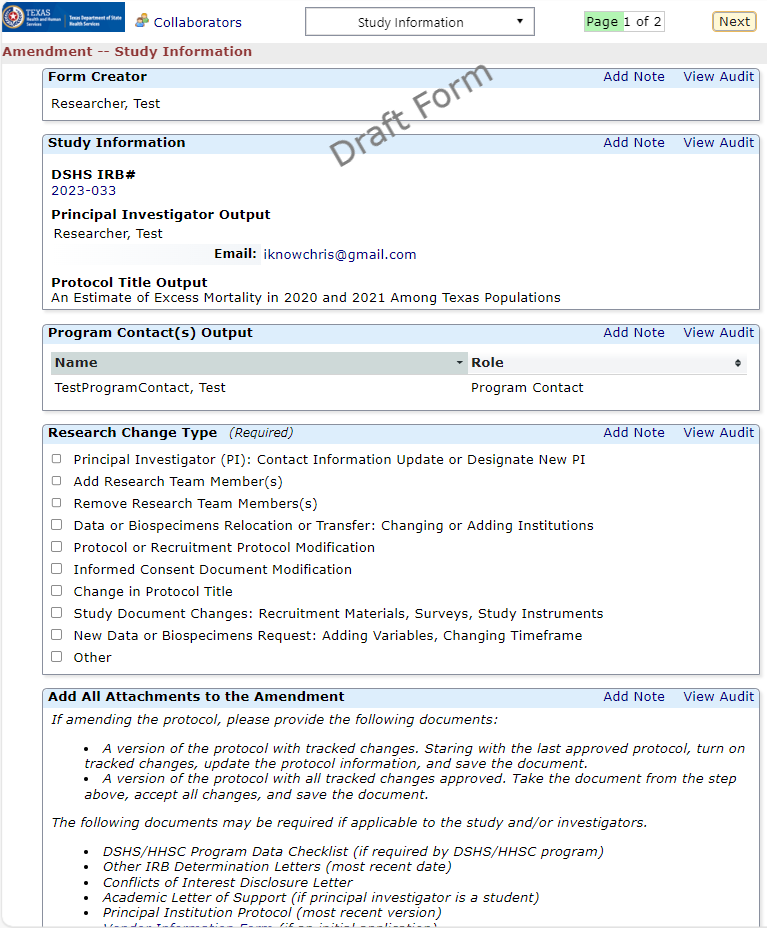
Amending a Protocol
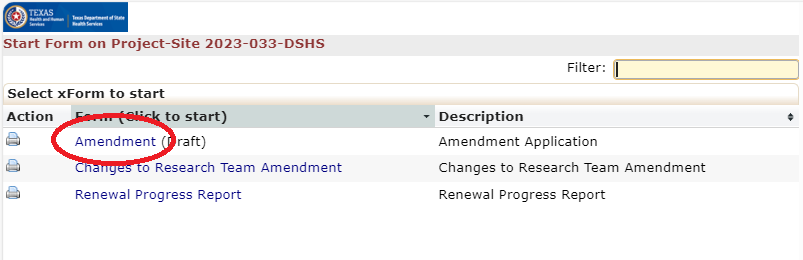
Amendment Forms:
- Use the non-legacy amendment xForm if the protocol was originally submitted in the DSHS OneAegis IRB system.
- Use the legacy amendment xForm if the protocol was originally submitted to DSHS before theDSHS OneAegis IRB system was created.
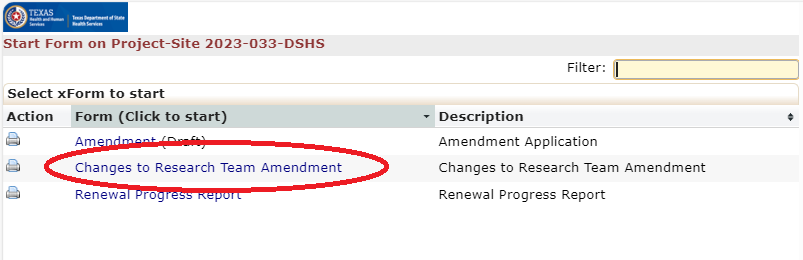
- Use the research team amendment xForm for all non-Principal Investigator research team changes.
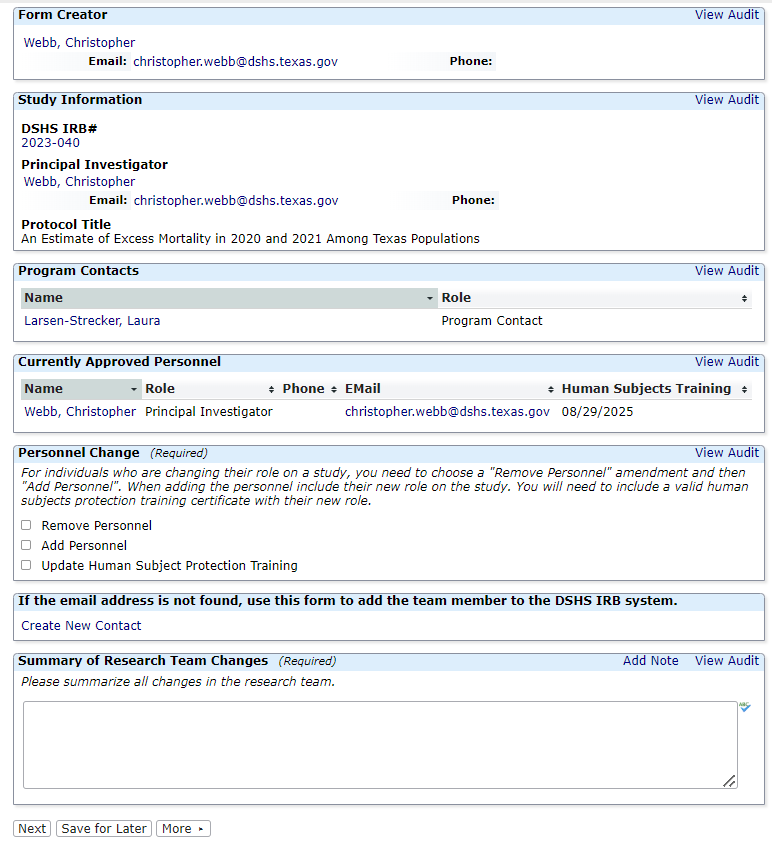
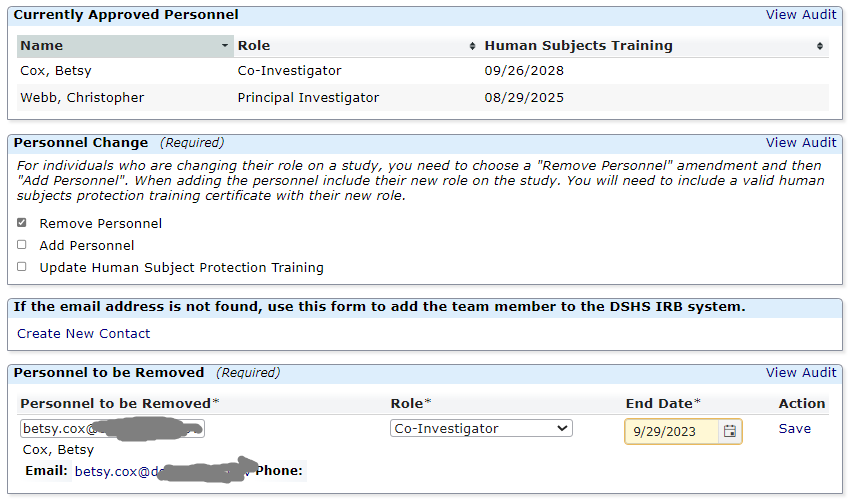
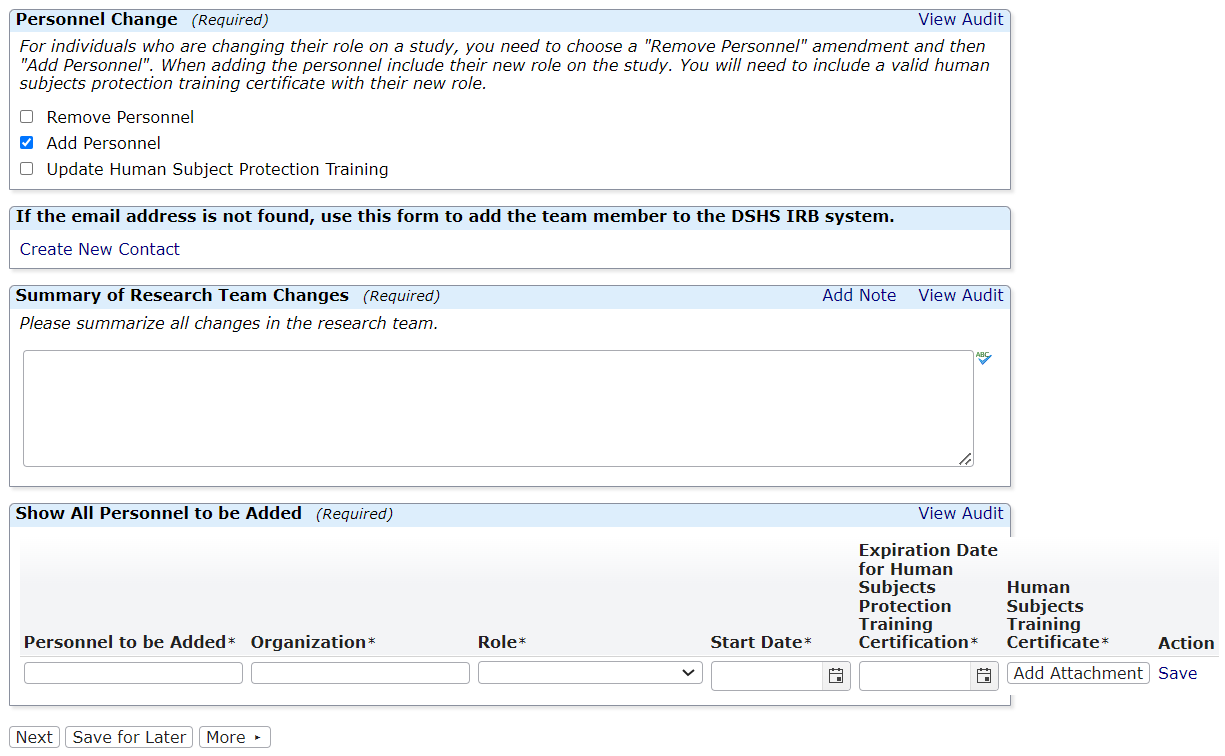
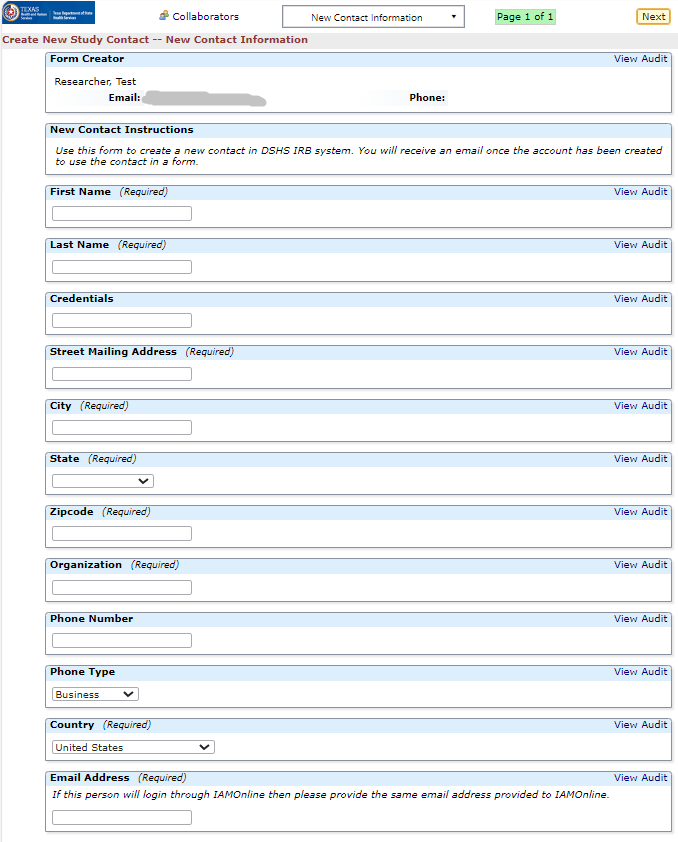
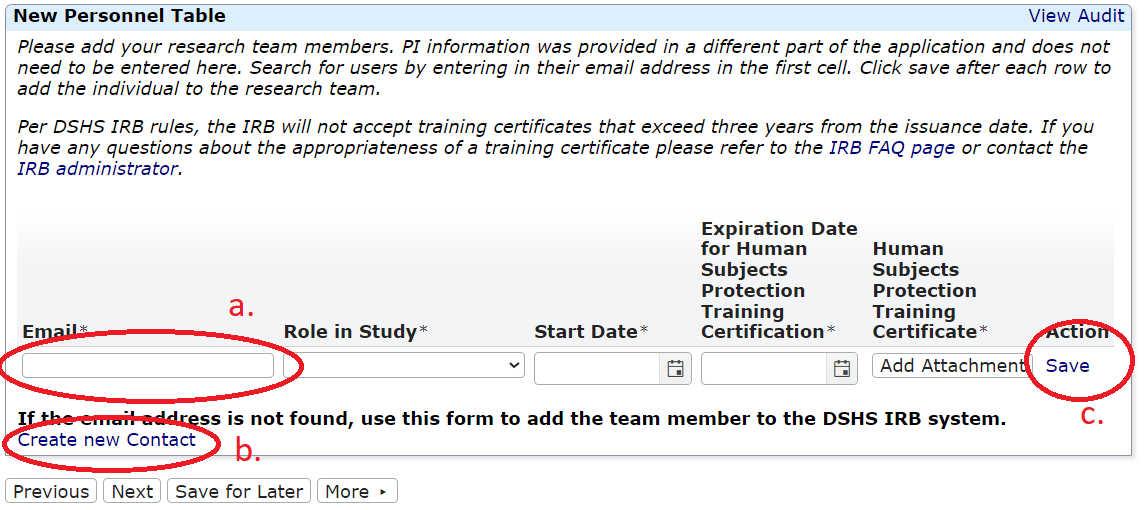
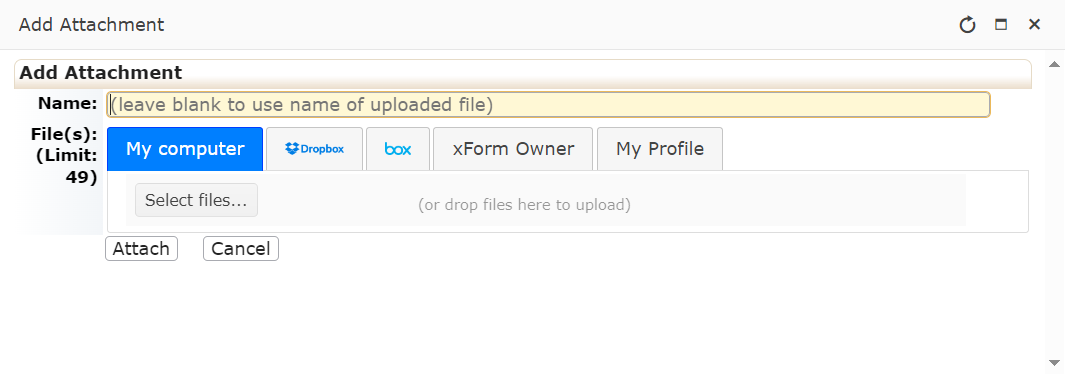
Research Team Amendments
This form is not appropriate for Principal Investigator (PI) Changes. Please submit a legacy or non-legacy amendment form for any PI change. You can use this application to update a PI or any other research team member's human subjects protection training certificate.
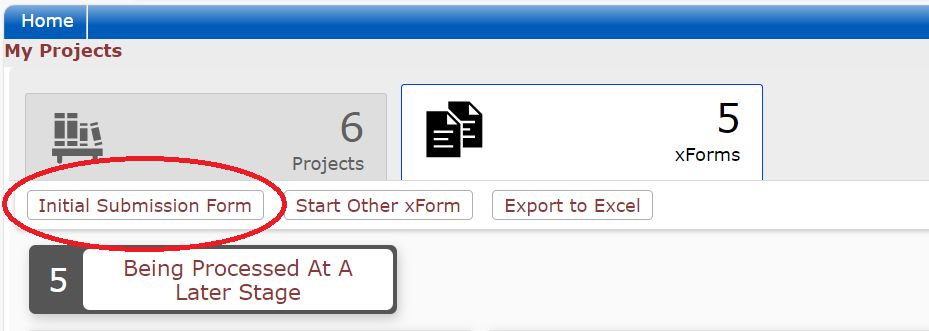
Initial Applications
All initial (new) applications will be stored and processed completely within the DSHS OneAegis IRB system. Researchers are encouraged to consult the IRB application guidance documents for assistance in how to write an application. This guidance will assist researchers in how to create and submit an application.
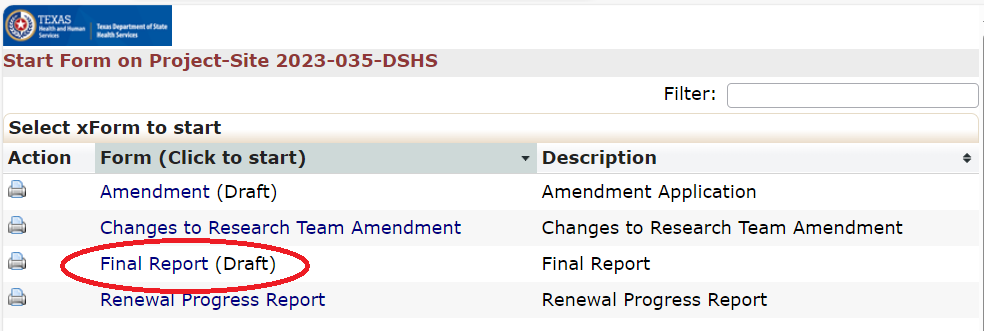
Final Report (Study Closure) Application
All Final Report applications will be stored and processed completely within the IRB system. Researchers are encouraged to consult the IRB submission guidance documents for assistance in how to write a final report application. This guidance will assist Principal Investigators (PI) in how to create and submit a Final Report application.
Note: Sometimes there are situations in which a PI is no longer associated with a protocol and cannot submit closure materials. Please contact the IRB administrator if a study needs to be reassigned to another individual who is not the current PI.
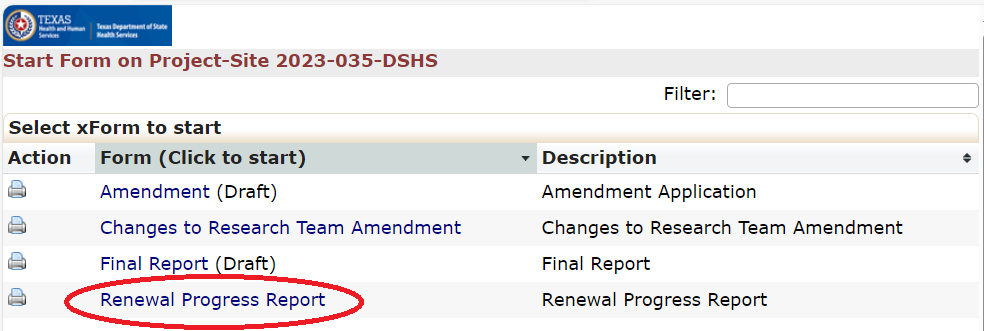
Renewal Progress Report
All Renewal Progress Report applications will be stored and processed completely within the DSHS OneAegis IRB system. Renewal Progress Reports should not include any modifications to the protocol. If changes are needed then researchers need to submit an amendment application.
Researchers are asked to review their research team and submit a Research Team Amendment if the research team has changed or if team members have expired human subjects protection training certificates.
Note: Sometimes there are situations in which a PI is no longer associated with a protocol and cannot submit renewal